Abstract
Follicular lymphoma (FL) is an indolent lymphoma of mature B-cells but may transform to a more aggressive histology, most commonly diffuse large B cell lymphoma. Recurrent mutations associated with transformation have been identified; however, biological predictors to guide initial therapy have remained elusive. We hypothesized that clonal heterogeneity and patient-specific immune responses would contribute to variable clinical outcomes and that understanding the complexity of the entire tumor "ecosystem" would allow more individualized matching of patients with specific therapies.
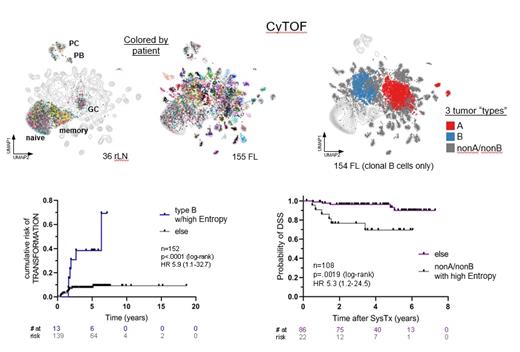
In prior ASH meetings, we presented preliminary analyses of B and T cell-focused phenotypic profiling of 155 newly diagnosed pre-treatment FL biopsy samples at single cell resolution by mass cytometry (CyTOF). These prior analyses unexpectedly revealed two distinct evolutionary trajectories which were independently reflected in both B and T cell compartments. One trajectory expectedly involved germinal center B cells (GCB); however, the other was more related to naïve/memory B-cells (NMB). Interestingly, cluster co-occurrence analysis suggested that the GCB and NMB trajectories were mutually exclusive of another and tended not to be found within the same tumor despite their high prevalences (χ 2 = 29.8, DF=1, p=4.8e-8; χ 2 test). Clustering analysis based on relative abundances of T cell subsets revealed 4 distinct immune patterns: Group 1 was characterized by naive T cells; Group 2 by T follicular helper (Tfh) cells; Group 3 by CD4+ regulatory T (Treg) and CD8 effector memory cells (CD8EM); and Group 4 by a diverse complement of naive, memory, and differentiated effector subsets.
We report here further analyses, now incorporating DNA mutational and clinical outcome information. Tumors were parsed into 3 types based on the phenotype of the majority (>50%) of tumor cells present in the diagnostic biopsy: Type A tumors dominated by GCB cells (28% of samples), type B tumors dominated by NMB cells (18% of samples), and type nonA/nonB tumors dominated by neither GCB nor NMB cells (54% of samples).
Type A tumors were significantly enriched for mutations in EZH2, TNFRSF14, and MEF2B, while no significant mutational associations were seen in type B and nonA/nonB tumors. Type B was significantly associated with increased risk of transformation, and when combined with a measure of intratumoral phenotypic diversity ("Entropy"), we found that type B tumors with high (above median) Entropy, representing 8.5% of all cases, exhibited a hazard ratio (HR) of 5.9 for transformation risk in comparison to all others combined (log-rank p<0.0001). Type B and high Entropy remained significant in multivariate analysis (p=0.043 and p=0.011, respectively), whereas high risk FLIPI score did not (p=0.962).
We also investigated survival outcomes using a sub-cohort of 108 patients who had received bendamustine + rituximab (BR) as their primary therapy. Despite the association of type B tumors with transformation risk, patients with type nonA/nonB tumors exhibited the poorest outcomes as measured by disease-specific survival (DSS; 5yr survival 78% vs 98% for all others combined, log-rank p=0.0241). Combining type nonA/nonB with high Entropy defined 20% of patients with significantly shorter DSS (HR 5.3, log-rank p=0.0019). In multivariate analysis, type nonA/nonB and high risk FLIPI score were significant (p=0.038 and 0.035, respectively), while high Entropy trended with inferior DSS (HR 2.8, 95% CI 0.76-10; p=0.123). Taken together, these data support that CyTOF-defined phenotypic subtypes of FL and intratumoral phenotypic diversity identify clinically significant subgroups at initial diagnosis and compare favorably against FLIPI score in predicting both risk of transformation and inferior DSS.
Freeman: Incyte: Honoraria; Seattle Genetics: Honoraria; Sanofi: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Amgen: Honoraria; Janssen: Honoraria, Speakers Bureau; Celgene: Honoraria; Teva: Research Funding; Abbvie: Honoraria; Roche: Research Funding. Sehn: Genmab: Consultancy; Novartis: Consultancy; Debiopharm: Consultancy. Savage: BMS: Consultancy, Honoraria, Other: Institutional clinical trial funding; Seattle Genetics: Consultancy, Honoraria; Astra-Zeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Other: Institutional clinical trial funding; Takeda: Other: Institutional clinical trial funding; Roche: Research Funding; Servier: Consultancy, Honoraria; Beigene: Other: Institutional clinical trial funding; Genentech: Research Funding. Craig: Bayer: Consultancy. Scott: Abbvie: Consultancy; AstraZeneca: Consultancy; Incyte: Consultancy; Celgene: Consultancy; NanoString Technologies: Patents & Royalties: Patent describing measuring the proliferation signature in MCL using gene expression profiling.; BC Cancer: Patents & Royalties: Patent describing assigning DLBCL COO by gene expression profiling--licensed to NanoString Technologies. Patent describing measuring the proliferation signature in MCL using gene expression profiling. ; Rich/Genentech: Research Funding; Janssen: Consultancy, Research Funding. Steidl: Trillium Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; AbbVie: Consultancy; Seattle Genetics: Consultancy; Curis Inc.: Consultancy; Bayer: Consultancy; Epizyme: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal